 首页>
生物试剂
首页>
生物试剂
SoluLyse Reagent for Bacteria
-
Higher yields of soluble proteins
-
No interference with affinity purification resins
-
Retain more protein activity
-
Low viscosity without enzyme or sonication.
-
Simple one-step protocol.
-
Compatible with protease inhibitors, salts, chelating agents and reducing agents
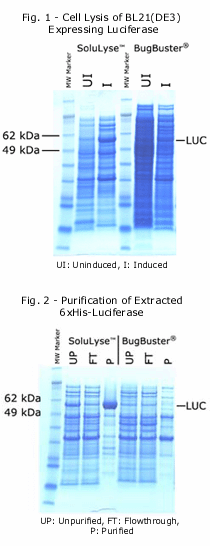
The SoluLyse™ Protein Extraction Reagent is a proprietary formulation of nonionic detergents that is optimized for the most efficient extraction of soluble proteins from bacterial cells. The lysis reagent allows perforation of bacterial cell wall without denaturing proteins, and there is no need for secondary treatment such as sonication or freeze-thaw.
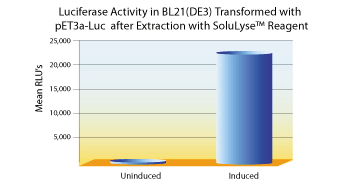
Side-by-side comparisons between SoluLyze and another leading commercial lysis reagents showed that SoluLyse yields about 10-fold more soluble proteins. In addition, SoluLyse reagent does not compromise protein activities in cell lysates.
The SoluLyse reagent is easy to use. Simply add it to a bacterial pellet and gently mix for 10 minutes. The cells will be disrupted and soluble proteins released. Insoluble cell debris can then be removed by centrifugation. SoluLyse is also useful for the preparation of high-purity inclusion bodies when the protein of interest is insoluble.
The SoluLyse Reagent is tested to be compatible with all popular purification methods, such as Ni and glutathione resins for purifying poly-His and GST-tagged proteins. The unique, mild formulation of SoluLyse offers minimized interference with the binding and elution processes and ensures superior purification results. In addition, the lysis reagent is compatible with a variety of additives such as protease inhibitors and reducing agents and may be readily dialyzed if needed.
Contents
- SoluLyse Protein Extraction Reagent is supplied in 1X solution in 50mM NaHPO4, pH 7.4.
- SoluLyse Reagent in Tris Buffer is supplied in 1X solution in 20 mM Tris HCl, pH 7.5.
Storage
Store at room temperature or +4ºC upon receipt. Stable for 2 years when stored properly.
Muscadine grape skin extract inhibits prostate cancer cells by inducing cell-cycle arrest, and decreasing migration through heat shock protein 40.
Ignacio, D. N.,et al (2019) Heliyon. 5(1): e01128.Link
Interplay of Klebsiella pneumoniae fabZ and lpxC Mutations Leads to LpxC Inhibitor-Dependent Growth Resulting from Loss of Membrane Homeostasis.
Mostafavi, M.,et al (2018) mSphere. 3(5): e00508-18. Link
An open library of human kinase domain constructs for automated bacterial expression.
Albanese, S. K.,et al (2018) Biochemistry. 57(31): 4675-4689. Link
Anacardic acid inhibits pancreatic cancer cell growth, and potentiates chemotherapeutic effect by Chmp1A -ATM - p53 signaling pathway.
Park, M., et al (2018) BMC Complement. Altern. Med. 18(1):71.Link
Multicenter Performance Assessment of Carba NP Test.
Cunningham, S. A.,et al (2017) J. Clin. Microbiol. 55(6): 1954-1960. Link
Characterization of an Acinetobacter baumannii lptD Deletion Strain: Permeability Defects and Response to Inhibition of Lipopolysaccharide and Fatty Acid Biosynthesis.
Bojkovic, J., et al (2016) J. Bacteriol. 198(4): 731-741. Link
Genomic mechanisms for cold tolerance and production of exopolysaccharides in the Arctic cyanobacterium Phormidesmis priestleyi BC1401.
Chrismas, N. A. M.,et al (2016) BMC Genomics. 17: 533. Link
Toxic Accumulation of LPS Pathway Intermediates Underlies the Requirement of LpxH for Growth of Acinetobacter baumannii ATCC 19606.
Richie, D. L.,et al (2016) PLoS. One. 11(8): e0160918. Link
RbsR Activates Capsule but Represses the rbsUDK Operon in Staphylococcus aureus.
Lei, M. G.,et al (2015) J. Bacteriol. 197(23): 3666-3675. Link
Protein-Peptide Arrays for Detection of Specific Anti-Hepatitis D Virus (HDV) Genotype 1, 6, and 8 Antibodies among HDV-Infected Patients by Surface Plasmon Resonance Imaging.
Villiers, M-B.,et al (2015) J. Clin. Microbiol. 53(4): 1164-71. Link
MicroRNA miR-182 cluster mediated modulation of RECK without changes in cell surface membrane type-1 matrix metalloproteinase (MT1-MMP).
Silva, M.,et al (2015) Am. J. Cancer Res. 5(9): 2918-2928. Link
Genlantis位于美国加利福尼亚州的圣地亚哥,是基因治疗系统公司的分公司。Genlantis致力于体内和体外诊断蛋白表达工具的研发及高效传递技术的开发。Genlantis为全球的生命科学研究客户生产研发生物学试剂,包括基因传递和质粒DNA的转染试剂(GenePORTER®),哺乳动物细胞siRNA转染试剂(GeneSilencer®),原代神经元细胞的优化转染试剂(NeuroFECT®)和原代神经细胞的转染试剂(NeuroPure®)。此外,我们还是Tap Express®和Xi-Clone® Instant Enzymeless PCR克隆技术的专利持有者。这些技术使得科学家们可以同时设计成百上千的蛋白,还可把成本控制在理想的范围内;可以使科学家们由复杂的细菌感染比如疟疾,肺结核,炭疽,天花病毒,衣原体,螺杆菌等快速的制备出相应的蛋白。
 会员登录
会员登录.getTime()%>)
 购物车()
购物车()
 成功收藏产品
成功收藏产品